End of assignment!
Friday, 8 July 2011
From the periodic table, there is an invisible "staircase". Above the "staircase" are elements that are non-metal, and below it are elements that are metal. Sulfur is found above the "staircase", therefore it's a non-meatl. Sodium is found below the "staircase" , therefore it's a metal.
Besides referring to the periodic table to know that sodium is a metal and sulfur is a non-metal as the elements in Group (I), (II) and (III) of the periodic table is metal and Group (IV),(V),(VI) and (VI) from the periodic table is non-metal. We could also refer to the number of electrons of the element.
Sodium (2.8.1) is a metal because it will lose 1 electron to complete the valence shell( to make it stable) and conduct electricity. Sulfur(2.8.6) is a non-metal because it will gain 2 electrons to complete the valence shell (to make it stable) and it does not conduct electricity.
Additionally, Group (III) is also known as transition metal or coloured metals. Group (0)/group (III) is known as the noble gas as they can stabilise themselves.
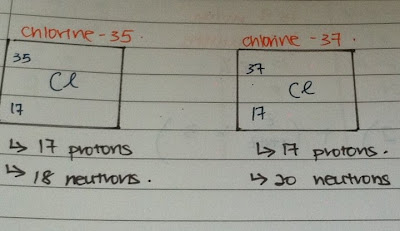 Isotopes are atoms of the same element with the same number of protons but different number of neutrons. Isotopes of the same element have the same number of electrons. thus, isotopes have the same proton number but different nucleon number. Most element that commonly occur are made up of isotopes. For an example. chlorine consists of two isotopes as what you can see from the picture above. A sample of chlorine gas consists of 75% chlorine-35 and 25% chlorine-37. The neutron number is added together then divided into 2.
Isotopes are atoms of the same element with the same number of protons but different number of neutrons. Isotopes of the same element have the same number of electrons. thus, isotopes have the same proton number but different nucleon number. Most element that commonly occur are made up of isotopes. For an example. chlorine consists of two isotopes as what you can see from the picture above. A sample of chlorine gas consists of 75% chlorine-35 and 25% chlorine-37. The neutron number is added together then divided into 2.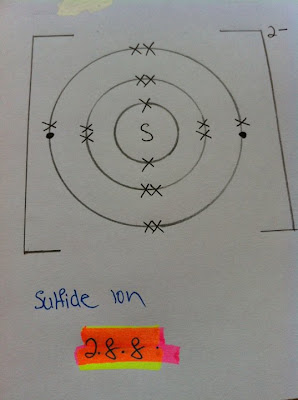
Sulfur atom needs 2 more electrons in the outermost shell to be stabilized. Thus, sulfide ion will be negatively charged. The number of electrons take by 2 for the third shell to be full but the proton number did not change.

Sodium gives away an electron and becomes positively charged. Then number of electron has decrease by one but the number of proton did not change. After losing an electron at the outermost shell, sodium will be stabilize. It has eleven electrons but after giving away one electron, the total number of electron of a sodium ion is 10. The outermost shell have only 1 electron is because the first and second shell is full. The first shell can only have two electron maximum and 4 pairs of electron for the second and third shell.
 Sodium has eleven electrons ( 2.8.1). The first and the second shell is full, therefore the third shell (outermost) have 1 electron. The first shell can only have two electrons and the maximum number of electrons in the second and third shell is eight.
Sodium has eleven electrons ( 2.8.1). The first and the second shell is full, therefore the third shell (outermost) have 1 electron. The first shell can only have two electrons and the maximum number of electrons in the second and third shell is eight.
rose |
0 rose(s) on your door

 Name: Nicole
Name: Nicole Tagboard here.
Tagboard here.
 Layout: Lost Days
Layout: Lost Days