Friday, 23 September 2011
mr tan i dont know why my blog is like that...
rose |
0 rose(s) on your door
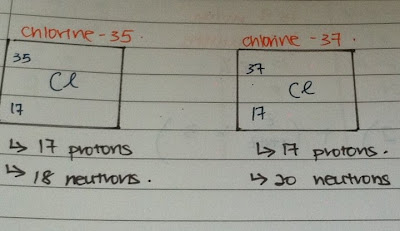 Isotopes are atoms of the same element with the same number of protons but different number of neutrons. Isotopes of the same element have the same number of electrons. thus, isotopes have the same proton number but different nucleon number. Most element that commonly occur are made up of isotopes. For an example. chlorine consists of two isotopes as what you can see from the picture above. A sample of chlorine gas consists of 75% chlorine-35 and 25% chlorine-37. The neutron number is added together then divided into 2.
Isotopes are atoms of the same element with the same number of protons but different number of neutrons. Isotopes of the same element have the same number of electrons. thus, isotopes have the same proton number but different nucleon number. Most element that commonly occur are made up of isotopes. For an example. chlorine consists of two isotopes as what you can see from the picture above. A sample of chlorine gas consists of 75% chlorine-35 and 25% chlorine-37. The neutron number is added together then divided into 2.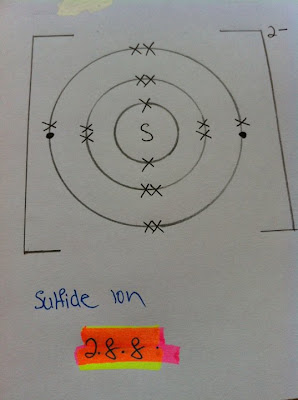

 Sodium has eleven electrons ( 2.8.1). The first and the second shell is full, therefore the third shell (outermost) have 1 electron. The first shell can only have two electrons and the maximum number of electrons in the second and third shell is eight.
Sodium has eleven electrons ( 2.8.1). The first and the second shell is full, therefore the third shell (outermost) have 1 electron. The first shell can only have two electrons and the maximum number of electrons in the second and third shell is eight.